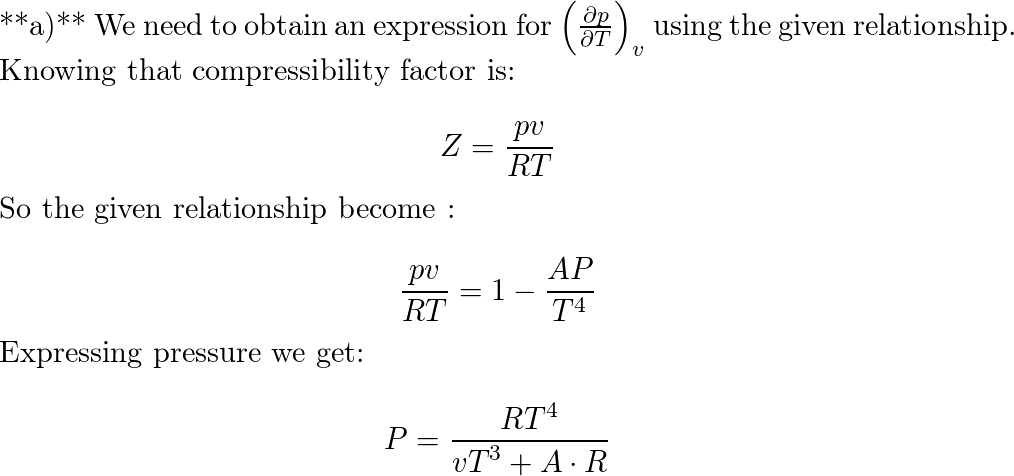- Home
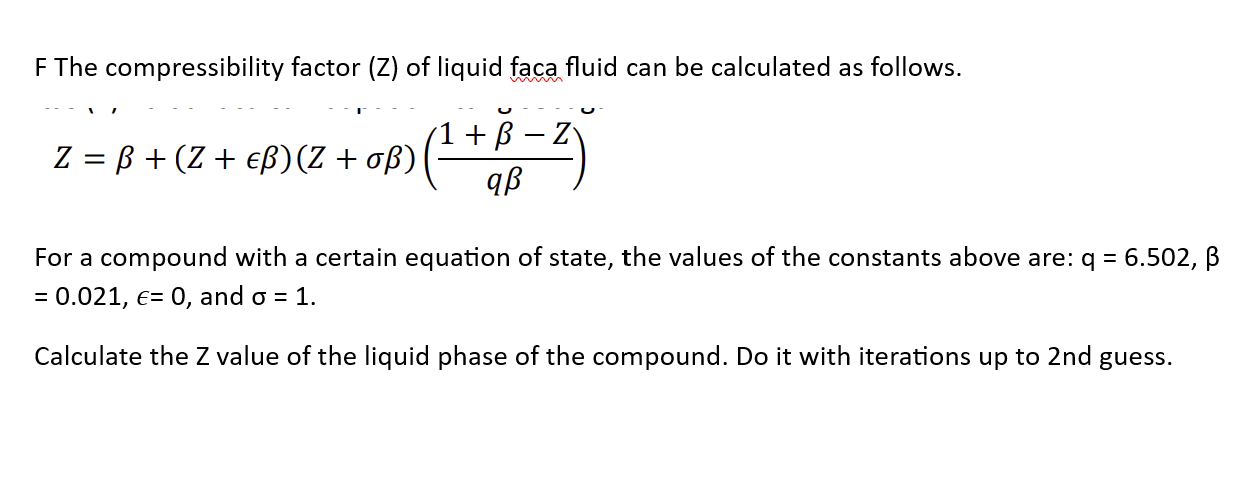
- compressibility factor equation
- 2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1.90 and 200 atm is 1.10.A certain mass of Noccupies a volume of 1
2) 1:12:15 (3) 12:15: Jals (4) 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1.90 and 200 atm is 1.10.A certain mass of Noccupies a volume of 1
4.8 (737) · $ 15.99 · In stock
Click here:point_up_2:to get an answer to your question :writing_hand:2 112153 1215 jals 42 5the compressibility factor for nitrogen at 330 k and 800
Click here👆to get an answer to your question ✍️ -2- 1-12-15 -3- 12-15- Jals -4- 2 5 The compressibility factor nitrogen 330 K and 800 atm is 1-90 and 200 atm is 1-10-A certain mass of Noccupies a volume of 1 dmat 330 Kand eoo atm calculate volume occupied by same cuany of gas 750 K and 200 atm- -1- 1 L -2- 2L -3- 3L

SOLVED: A gas at 350 K and 12 atm has a molar volume 12 per cent
Find the compressibility factor for nitrogen at. 2000 kPa, 1

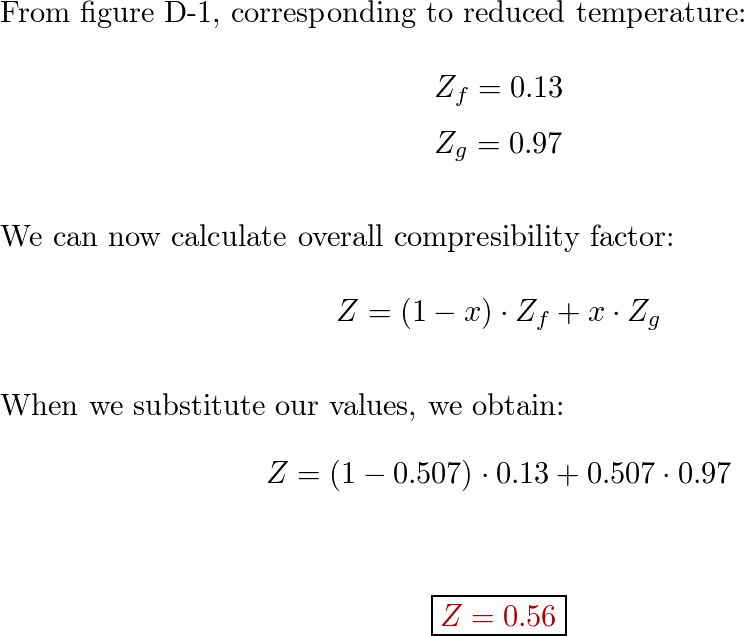
Solved Compressibility Chart 5.00 1.20 1.15 1.10 1.05 1.00

3.3: Real gas and compressibility factor - Engineering LibreTexts

Calculate the compressibility factor for a gas, if 1 mole of it
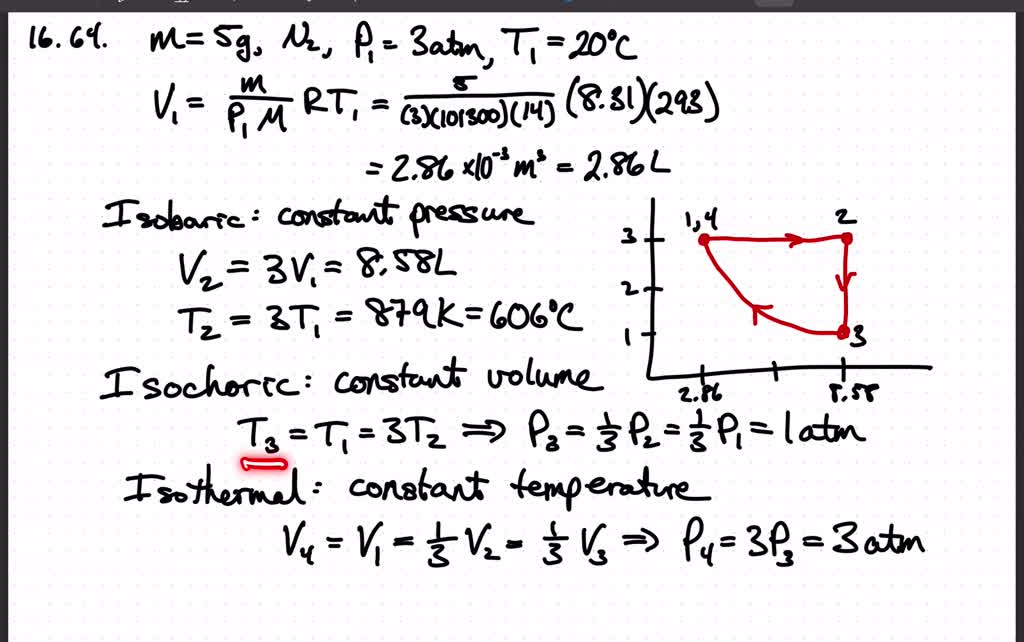
⏩SOLVED:Five grams of nitrogen gas at an initial pressure of 3.0

SOLUTION: Fluid mechanics problem exercises chapter 1 - Studypool

At total pressure P_1 atm and P_2 atm N_2O_4 is dissociated to an
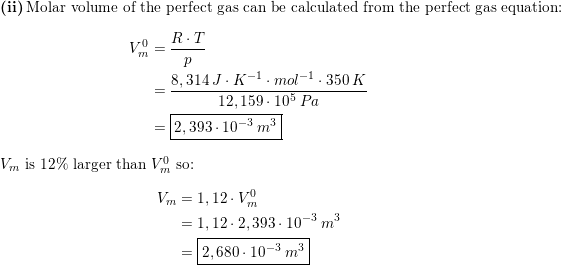
a) A gas at 250 K and 15 atm has a molar volume 12 per cent

Compression Factor Exam Problem using Molar Volumes - Fully

The compressibility factor of nitrogen at 400 K and 800 atm is

Three moles of a ideal gas at 200K and 2 0 atm pressure undergo
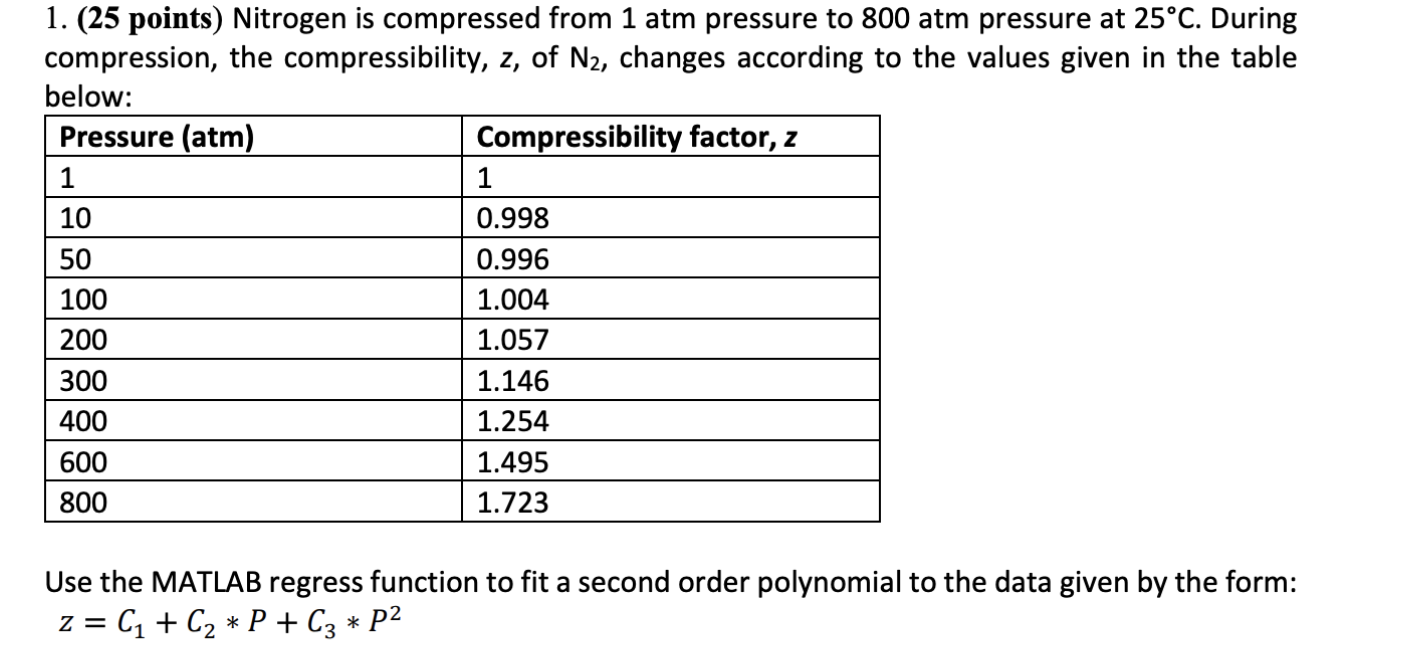
Solved 1. (25 points) Nitrogen is compressed from 1 atm

Compression Factor Exam Problem using Molar Volumes - Fully
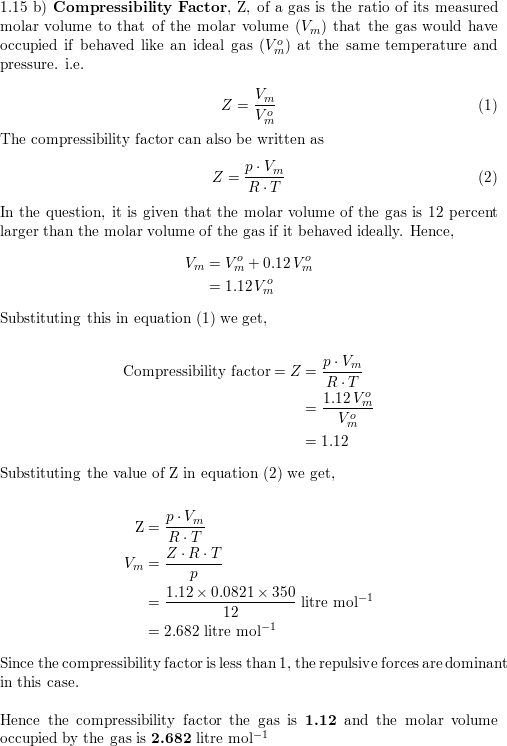
a) A gas at 250 K and 15 atm has a molar volume 12 per cent