- Home
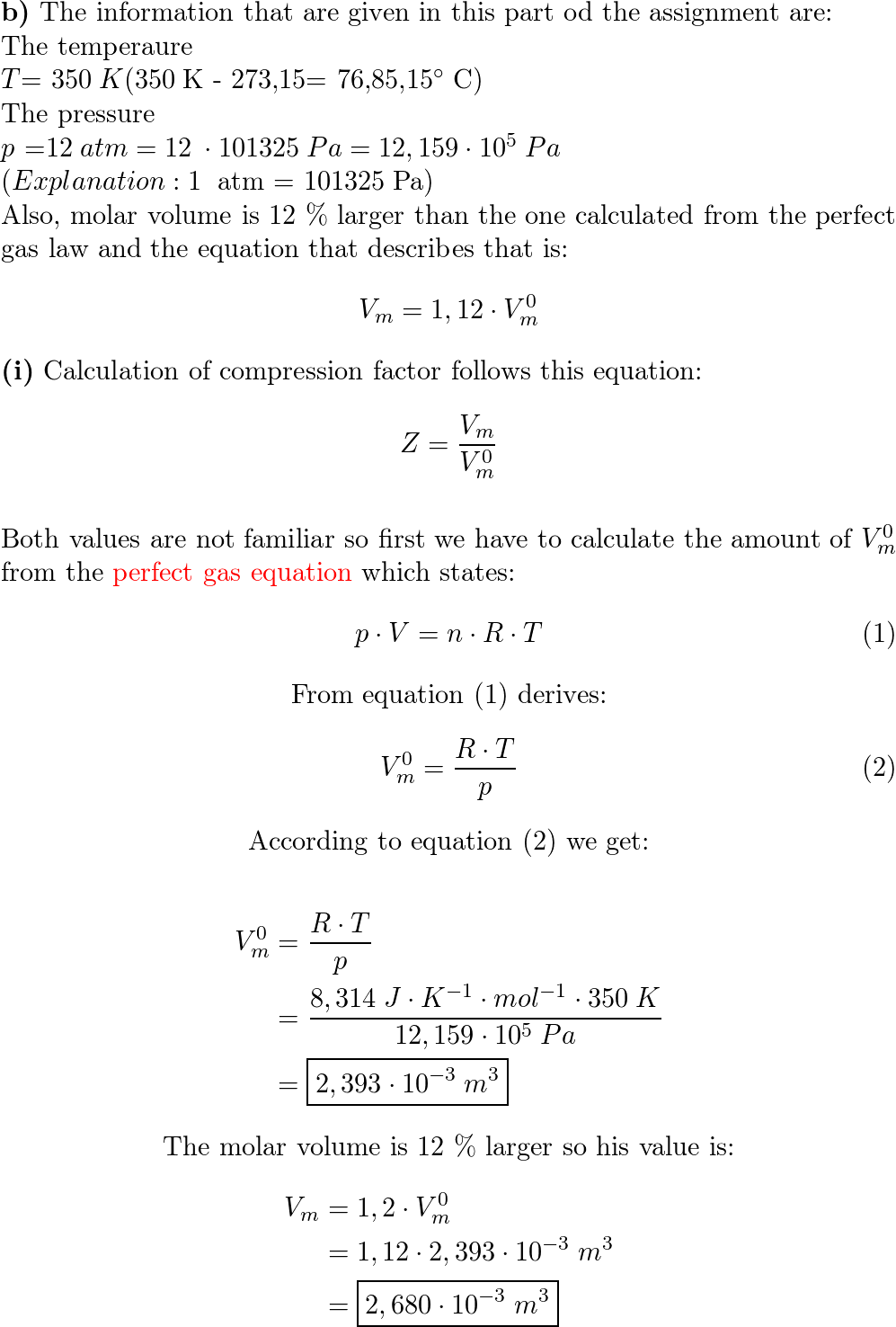
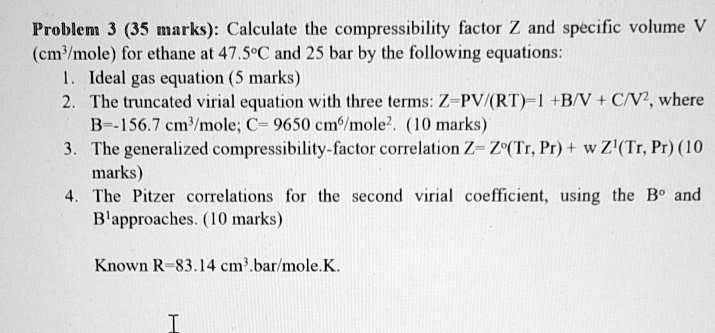
- compressibility factor equation
- What is the compressibility factor (Z) for 0.02 mole of a van der
What is the compressibility factor (Z) for 0.02 mole of a van der
4.6 (775) · $ 7.99 · In stock

atm. 6. What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of lam. Assume the size of gas molecules is negligible. Given : RT = 20

B-16. Ω What is the compressibility factor (Z) for 0.02 mole of a van der..
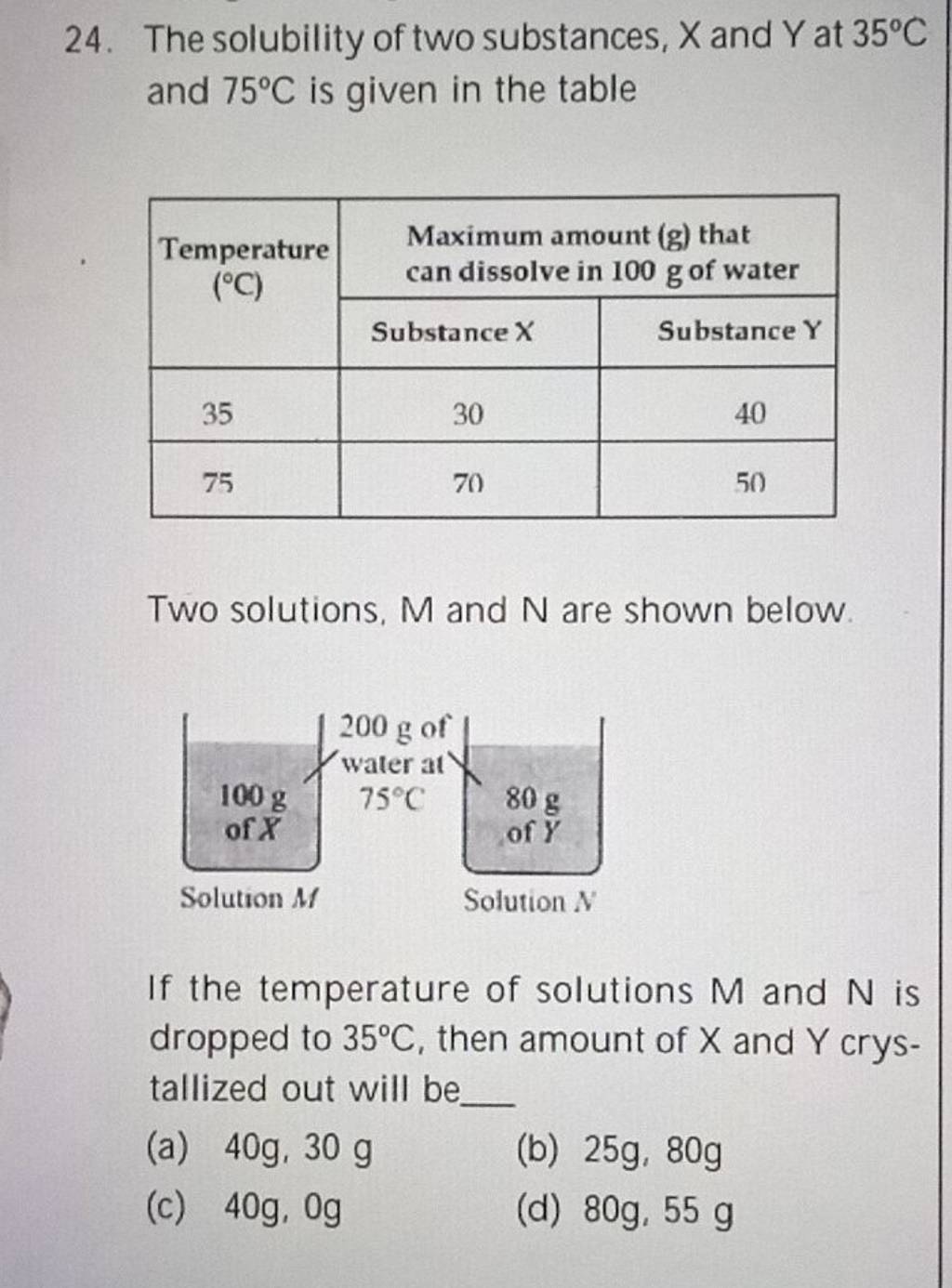
The solubility of two substances, X and Y at 35∘C and 75∘C is given in th..

Water in Petroleum and Petroleum Products

al Gases f.a What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. Given: RT =

Compressibility factor - Wikipedia

compressible flow related terms - Department of Mechanical and
58.7 Maximum mass of hydrogen is present in(1) 0.1 mol of CH1206(2) 1.5 mol of NH3(3) 22.4 L of H2S(g) at S.T.PSo(4) 0.5 g molecule of CeH

Van Der Waals Equation - an overview

Compressibility Factor Calculator - File Exchange - MATLAB Central

Compressibility Factor - an overview

ODPOOD B-76.& What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. Given : RT =

Physical Chemistry OBJECTIVE, PDF, Atomic Orbital

jo 22] What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible.