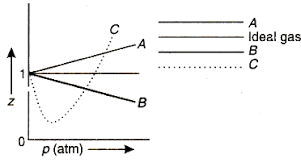
If Z is a compressibility factor, van der Waals equation at low
4.5 (278) · $ 5.50 · In stock
Solution For If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 1: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 2: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 3: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 4: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as

Non-ideal behavior of gases (article)

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced

At a high pressure, the compressibility factor (Z) of a real gas is us
For one mole of a van der Waals gas when b0andT300K the PVvs1Vplot
Thermo] Derivation of compressibility factor vs reduced pressure

⏩SOLVED:If Z is a compressibility factor, van der Waals equation

SOLVED: If PR=3, TR=2.0, the compressibility factor (Z) is equal

Non-Ideal Gas Behavior Chemistry: Atoms First

Which of these are correct? A) Z, compressibility factor, low

⏩SOLVED:If Z is a compressibility factor, van der Waals equation

If `Z` is a compressibility factor, van der Waals' equation at low