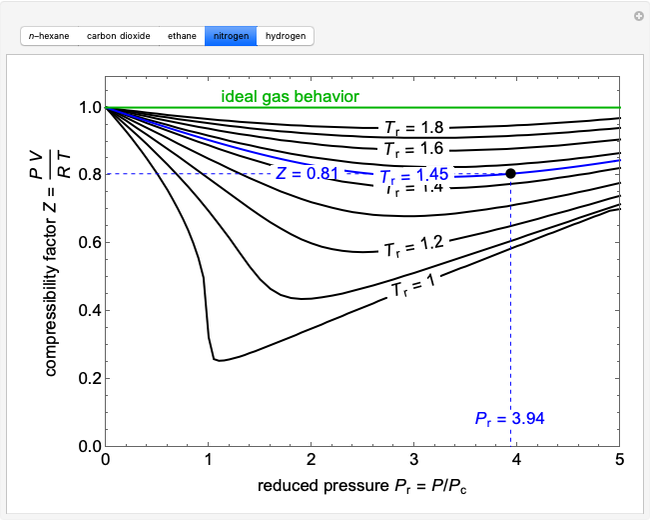
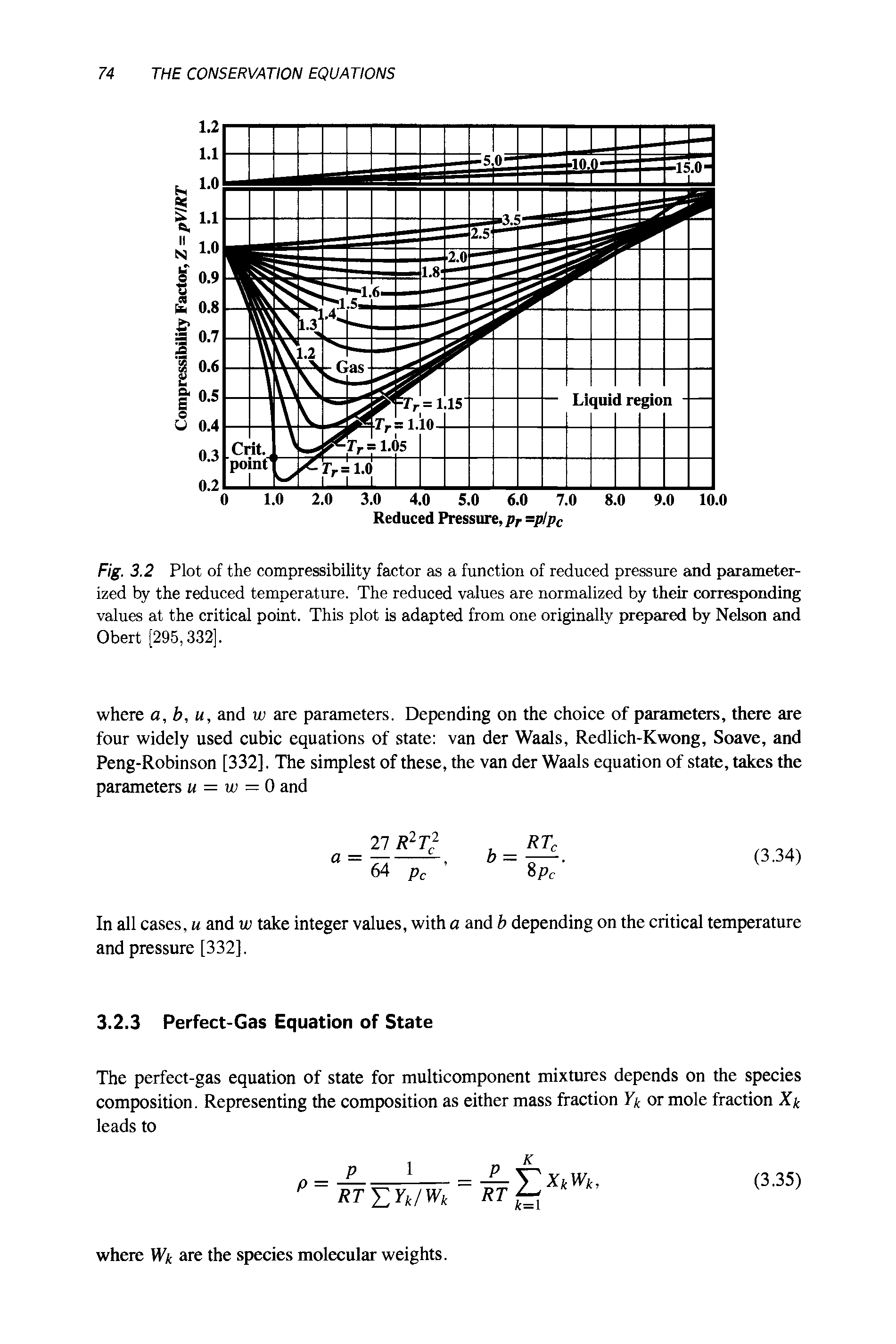
Compressibility factor Z = PV / nRT is plotted against pressure as
4.5 (73) · $ 10.99 · In stock
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2

1.1: Thermodynamic Variables and Equations of State - Chemistry LibreTexts

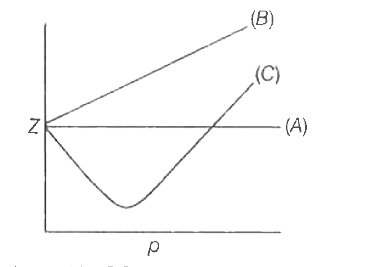
Compressibility factor Z is plotted against pressure p for four different gases A , B , C & D. The correct order of critical temperature of the gasesA. A>B>C>DB. B>A>C>DC. D
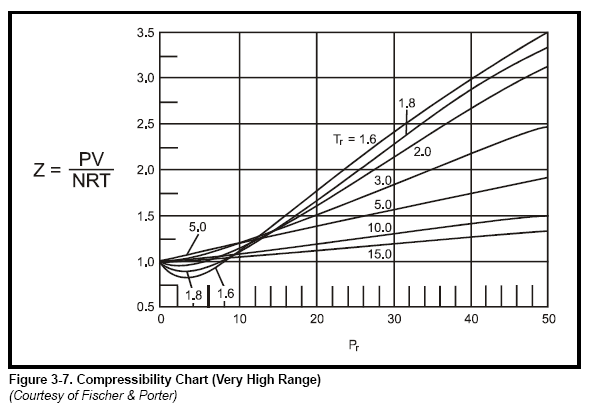
Chapter 3 - Physical Properties of Fluids: Gas Compressibility Factor
Telugu] The variation of compressibility factor (Z) with pressure (p
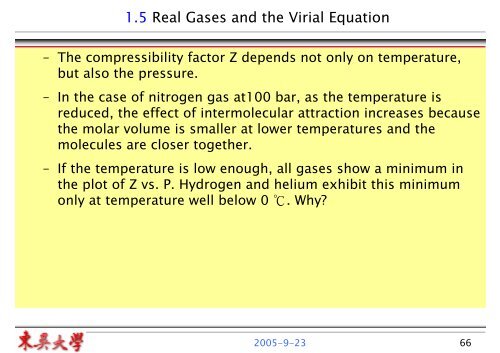
1.5 Real Gases and the Virial Equation - Mail
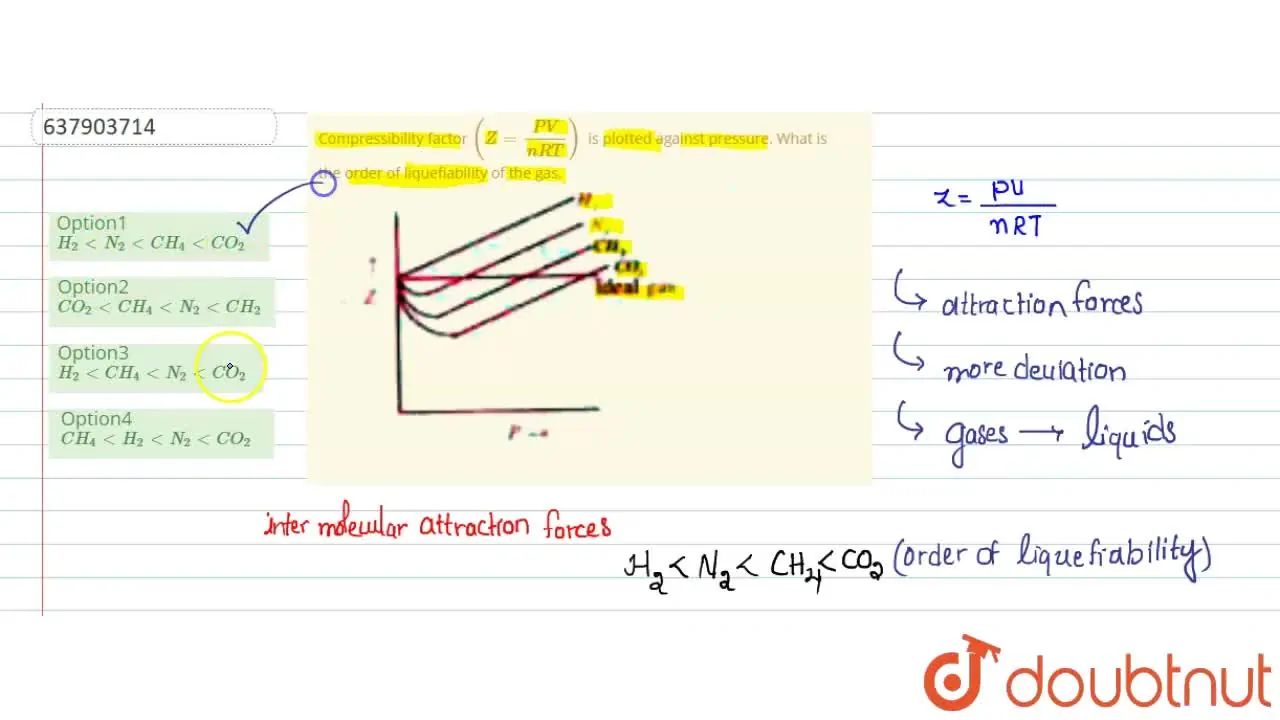
Telugu] Compressibility factor (Z = (PV)/(nRT)) is plotted against p

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

A real gas M behaves almost like an ideal gas. Graph 1 is obtained by plotting volume, V against temperature, T for x mol of gas M at pressure, P_1. a. Suggest

For one mole of a real gas, curves are plotted under different conditions the same temperature as shown in diagram: slope = 102 2463 C (In low pressure region) RT slope =

Van der Waals equation - Wikipedia
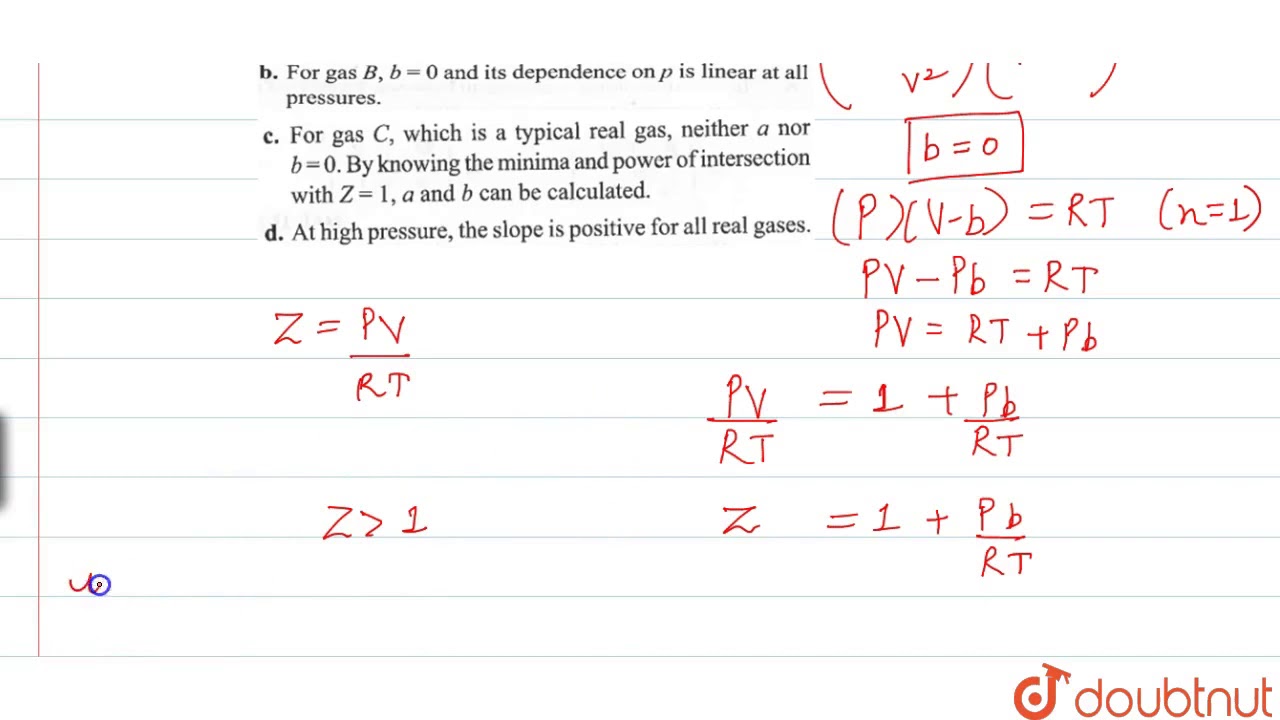
The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Explain the shape of graph obtained between pressure P and 1/v for perfect gas at constant temperature? - Quora
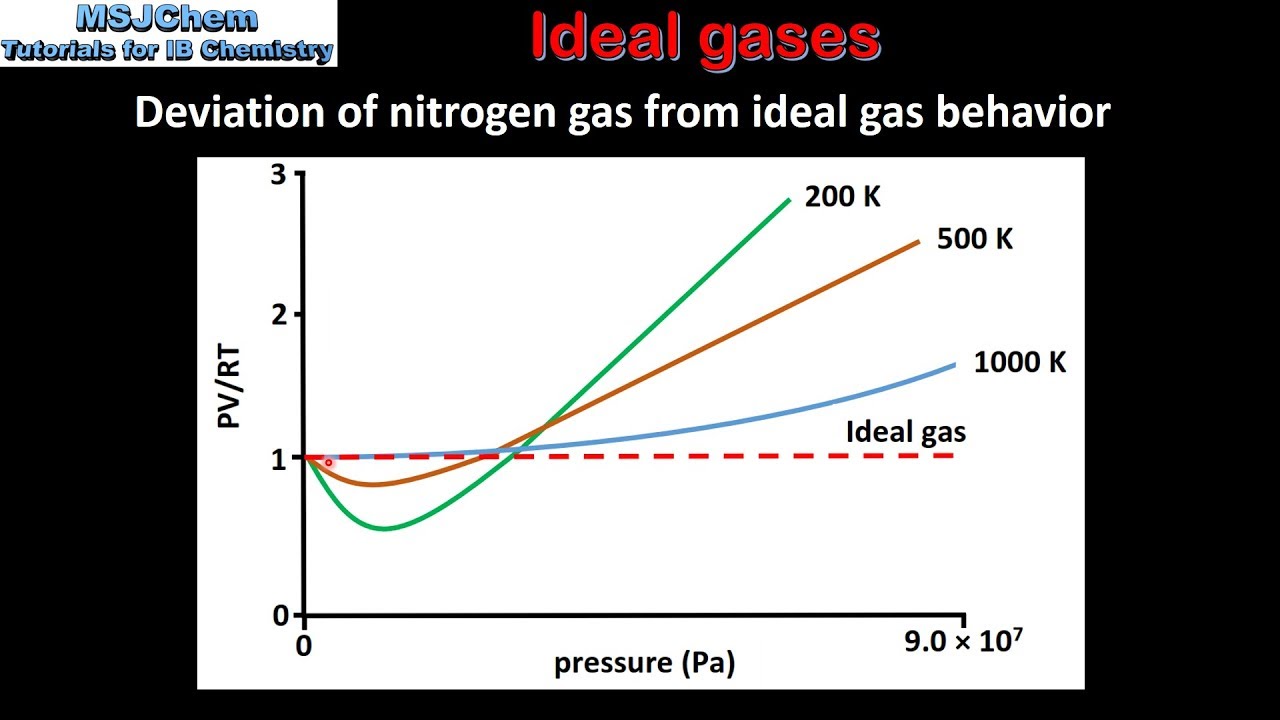
1.3 Deviation from ideal gas behaviour

Gas Compressibility - an overview